Abstract
Background: JAK2 inhibitors (JAK2i) are now part of the expanding therapeutic armamentarium for myeloproliferative neoplasms (MPNs), specifically for myelofibrosis (MF) and polycythemia vera (PV). In this regard, ruxolitinib has paved the way with its FDA approval in 2011. There is general agreement on the efficacy of JAK2i in controlling splenomegaly and constitutional symptoms whereas there is limited evidence for value in modifying disease natural history, including impact on progression into acute myeloid leukemia (AML), also referred to as blast phase (BP) MPN. We have previously published our pre-ruxolitinib era experience with MPN-BP and underscored its dismal prognosis with median survival of 3.6 months and 3-year survival rate of only 1%, in the absence of treatment response and consolidation with allogeneic hematopoietic stem cell transplant (AHSCT) (Tefferi et al.Leukemia 32:1200, 2018). In the current study, we describe our more recent experience, subsequent to the FDA approval of ruxolitinib.
Methods: The current study was conducted under an institutional review board approved minimum risk protocol that allowed retrospective collection and analysis of data from patients with BP-MPN who were seen at the Mayo Clinic, Rochester, MN, USA between 12/16/2011 and 5/27/2021. Diagnoses of specific MPN variants and post-MPN AML was according to the 2016 WHO criteria (Arber et al. Blood2016;127:2391) Estimates for chemotherapy-induced complete remission (CR), CR with incomplete blood count recovery (CRi), and morphologic leukemia-free state (MLFS) rates were according to conventional criteria (Dohner et al. Blood 2017;129:424). Clinical and laboratory data, including cytogenetic and molecular information, were collected from patients at the time of leukemic transformation. Conventional statistical methods were applied using JMP Pro 16.0.0 software (SAS Institute, Cary, NC, USA).
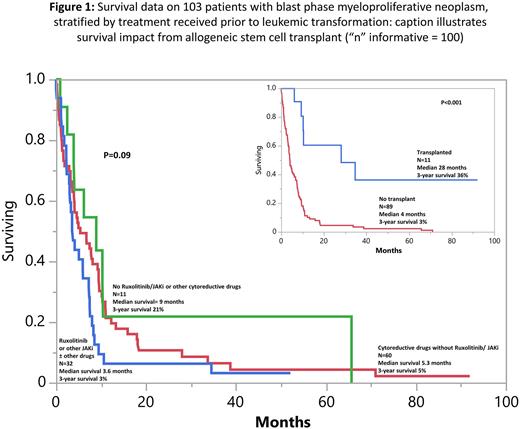
Results: Among 103 study patients (median age 70 years, range 37-89; 52% males), MPN variant prior to AML transformation was primary MF (PMF) in 35% and post-PV/ET MF in 65%. Karyotype was available in 97 patients and revealed monosomal karyotype or monosomy 7 in 35 (36%), complex karyotype, non-monosomal in 18 (19%), normal karyotype in 17 (18%) and other abnormalities in 27 (28%). Driver mutation distribution ("n” evaluable = 83) was JAK2 67%, CALR 7%, MPL 6% and triple-negative 1%. Median time from MPN diagnosis to leukemic transformation was 7.5 years. Thirty-two (31%) patients had received ruxolitinib and/or other JAK2i ± other cytoreductive drugs, 60 (58%) had received cytoreductive drugs (often hydroxyurea) but not JAK2i, and 11 (10%) had received neither JAK2i or other cytoreductive drugs, prior to leukemic transformation; patients with MPN-BP, developing in the context of the three treatment groups, were similar in their phenotype, karyotype and mutation distribution (Table to be shown at meeting).
First-line MPN-BP therapy included intensive chemotherapy (n=35), hypomethylating agents (HMA) with (n=12) or without (n=21) venetoclax, imetelestat (n=6) or supportive care in the remaining. AHSCT was documented in 11 patients. 71 patients were evaluable for response and included 11 (15%) with CR/CRi and 7 (10%) with MLFS. At the time of this writing, 96 (93%) deaths were documented. Among the 7 patients censored alive, 5 had received AHSCT, 4 of whom had persistent bone marrow blasts (8-16%) at time of transplant. There was no significant difference (p=0.09) in survival, based on pre-AML exposure to JAKi ± other cytoreductive drugs vs cytoreductive drugs not including JAKi vs neither JAKi or other cytoreductive drugs (Figure 1); the corresponding median survivals (3-year survival rates) were 3.6 months (3%), 5.3 months (5%), and 9 months (21%). By contrast, there was a favorable impact (p<0.01) from AHSCT with median survival (3-year survival rate of 28 months (36%) vs 4 months (3%) without transplant (Figure 1). In multivariable analysis, absence of AHSCT (p<0.01), older age (p=0.03) and thrombocytopenia (p<0.01) were associated with inferior survival.
Conclusions: Pre-AML exposure to ruxolitinib or other JAKi might not modify the phenotype, mutational landscape or the dismal prognosis associated with MPN-BP. AHSCT was able to secure long-term survival in some patients with MPN-BP and should thus be aggressively pursued, even in the absence of CR/CRi/MLFS.
Disclosures
Al-Kali:Astex: Other: research support to institution. Begna:Novartis: Honoraria; ImmunoGen: Research Funding. Litzow:Abbvie: Research Funding; Amgen: Research Funding; Astellas: Research Funding; Novartis: Research Funding; Syndax: Research Funding; Jazz: Consultancy; Actinium: Research Funding; Pluristem: Research Funding; Biosight: Other: Data Monitoring Board. Mangaonkar:Bristol Myers Squibb: Research Funding. Patnaik:Kura Oncology, Stemline Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal